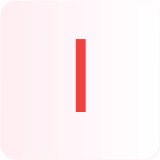
Wait!

Before You Leave... Find Your Perfect Job!
Are you sure you don't want to discover the perfect job opportunity? At JobPe, we help you find the best career matches, tailored to your skills and preferences. Don’t miss out on your dream job!